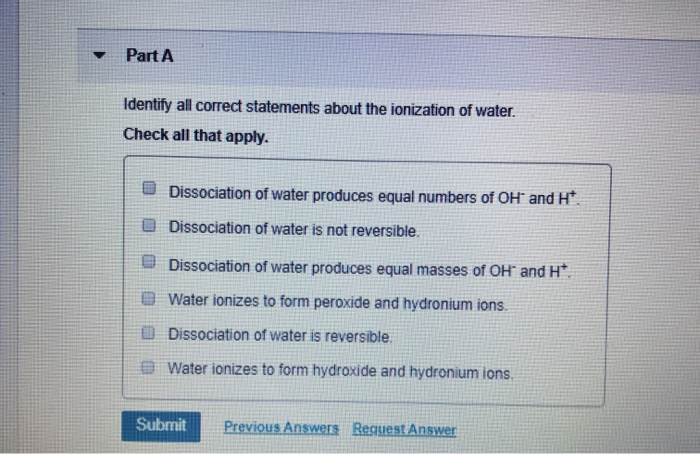
Neutral solutions contain no H3O ions. Identify all correct statements about the ionization of water.
Solved Part A Identify All Correct Statements About The Chegg Com
Oxygen has a larger first ionization energy than fluorine.

. - Core electrons are the innermost electrons of. Dissociation of water is not reversible. CHEM LEARNSMART CH8 Which statement correctly describes core electrons.
Biology questions and answers. H 2 Ol H 2 Ol H 3 O aq OH-aq or 2 H 2 Ol H 3 O aq OH-aq. Dissociation of water is not reversible.
Check all that apply. H_3O OH 1003 times 107. When pure liquid water is in equilibrium with hydronium and hydroxide ions at 25 C the concentrations of the hydronium ion and the hydroxide ion are equal.
The equilibirum expression for the above reaction is written below and is treated mathematically like all equilibrium expressions. Dissociation of water produces equal numbers of OH- and H. Identify all correct statements about the ionization of water.
Dissociation of water produces equal numbers of OH and H. Ionization energies are always negative quantities. Water ionizes to form hydroxide and hydronium ions.
A water ionizer makes two types of water at the same time because of the way the ionization process works. Sodium chloride NaCl dissolves when water molecules continuously attack the NaCl crystal pulling away the individual sodium Na and chloride Cl ions. Waters high surface tension.
Identify all correct statements about the ionization of water. Neutral solutions contain no OH ions. Water ionizes to form peroxide and hydronium ions.
Check all that apply. 3Water ionizes to form hydroxide and hydronium ions. This is true of any conjugate pair of acid and base Salts of Acids and Bases ν When an acid and a base undergo an exchange reaction the result is a salt and water.
Check all that apply. Water ionizes to form peroxide and hydronium ions. 1Dissociation of water is reversible.
2H2O H30 OH. Dissociation of water produces equal masses of OH and H. When there is collision between two water molecules self-ionization happensSelf-ionization of water is when two water molecules interact to form a hydronium ion and a hydroxide ion.
Water ionizes to form hydroxide and hydronium ions. Identify which of the following statements are true. Dissociation of water produces equal masses of OH and H.
Alkali elements are Lithium Li Sodium Na Potassium K Rubidium Ru Cesium Cs and Francium Fr occupying successive periods from first to seven. Check all that apply. This reaction is reversible and referred as autoionizationThis reaction happens because water is amphiprotic which means it can act as acid or base either the one.
The Auto-Ionization of Water K w. The second ionization energy of an atom is always greater than its first ionization energy. Part A Identify all correct statements about the ionization of water.
Two water molecules interact to form a hydronium ion and a hydroxide ion. Neutral solutions contain H3O and OH ions in equal concentrations. Dissociation of water produces equal numbers of OH and H.
1Dissociation of water is reversible. Which of the functional groups shown above is most likely to gain a proton and become positively. Drag the appropriate label under each illustration to identify the.
Dissociation of water produces equal numbers of OH- and H. Water ionizes to form peroxide and hydronium ions. Identify all correct statements about the ionization of water.
What does the term electron orbital describe. This nonstop attack continuous until the whole NaCl crystal disintegrates. During the ionization process the minerals in the water are separated from the bicarbonates using electromagnetism and a special membrane that allows ions which are charged particles to pass through it.
View CHEM LEARNSMART CHAPTER 8docx from GRADE 3 3 at University of Phoenix. Identify all correct statements about the ionization of water. NCERT Exemplar Chemistry Class 11 Chapter 7 Equilibrium helps you in understanding the basic concepts of equilibrium in an interactive manner.
Equilibrium is a very basic topic that is necessary for the students to understand in order to score well in the exams and to face bigger challenges in the. Dissociation of water is reversible. Water undergoes auto-ionization according to the following equation.
Dissociation of water produces equal numbers of OH and H. Click hereto get an answer to your question Which of the following statements isare correct about the ionic product of water. Water ionizes to form hydroxide and hydronium ions.
Check all that apply. Check all that apply. Francium is a radioactive element with very low half-life.
NCERT Exemplar Solutions Class 11 Chemistry Chapter 7 Free PDF Download. To understand this process at the molecular level we must apply the three steps we previously discussed. Water ionizes to form hydroxide and hydronium ions.
HXaq MOHaq MXaq H 2O acid base salt ν If a strong base is neutralized with a strong acid the resulting solution contains only the salt HClaq NaOHaq NaCl. Dissociation of water produces equal numbers of OH- and H. Dissociation of water is not reversible.
Identify all correct statements about the ionization of water. 2Dissociation of water produces equal numbers of OH- and H. Identify all correct statements about the ionization of water.
M label1636 Thus the number of dissociated water molecules is very small indeed approximately 2 ppb. A Dissociation of water produces equal masses of OH- and H. E Water ionizes to form peroxide and hydronium.
Alkali metals have a corresponding Noble gas ns 1 electronic configuration. Which of the following statements is true for neutral solutions in which water is the solvent. Dissociation of water produces equal masses of OH and H.
They occupy the first column of the periodic table.

Solved Identify All Correct Statements About The Ionization Chegg Com

Periodic Trends Electronegativity Ionization Energy And Atomic Radius Ionization Energy Chemistry Worksheets Classroom Tools

Ion Product Constant Chemistry Basics Science Chemistry Chemistry Notes
0 Comments